Chip maker AMS has unveiled a sensor-enabled NFC tag that could be used in a subdermal implant, enabling diabetes sufferers to read their glucose levels at any time by simply holding an NFC phone to a tiny device implanted under their skin — and replacing the need for finger pricking and dedicated glucose monitoring devices.
The SL13A is an ISO 15693 compliant tag that includes an on-board temperature sensor as well as an interface for an optional external sensor and can be read by NFC phones and RFID readers.
The device is able to operate in passive mode, where the tag harvests energy from the reader. That power can then be used to read the temperature or activate another sensor and transmit the result back to the reader.
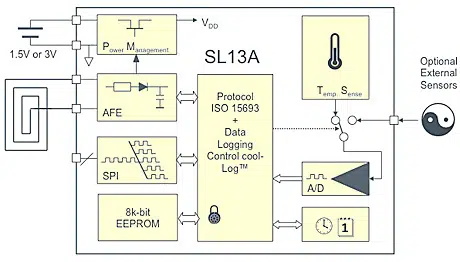
The SL13A can be used in cold chain tracking, where ambient temperature is periodically logged to ensure products are kept at the correct temperature throughout production and distribution.
“The most interesting scenario is in the medical area, for example, making it easier for patients to check their levels and potentially save medical bills and hospital costs by enabling people to track their situation from home and not necessarily need expensive clinical staff to monitor people,” AMS’ Oluf Alminde told NFC World.
“If you take diabetes as a use case, there are more than 360 million people in the world with diabetes,” he explained. “All of these people need to measure their blood glucose level, often several times a day. Right now, they take a sample of blood by pricking their finger and with their specialist equipment can measure their glucose level.
“Our idea is to implant the sensor along with our SL13 tag, which is of course batteryless, meaning all the patient needs to do is bring their NFC phone to the part of their body where it is implanted, which will activate the tag and also provide the power — they can then take the reading using an app.”
“The information could then be stored in the phone or even sent automatically to the hospital, clinic or doctor,” he added. “We’re already talking to companies in this area and actually working on this kind of solution, so you can expect to see this sort of thing in a few months time.”
ISO 15693 is not supported by all NFC devices as it is not one of the four tag types mandated by the NFC Forum. It is supported by Android, however, where it is referred to as NFC-V and AMS tells NFC World that it has found that all Android NFC devices will work with its tag.
Next: Visit the NFCW Expo to find new suppliers and solutions